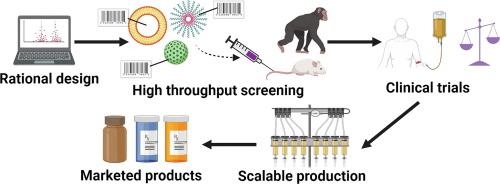
Clinical translation is the process of transforming new scientific knowledge into patient-friendly treatments and therapies. This procedure is crucial for creating novel, efficient remedies for illnesses and disorders as well as for enhancing patient care. Basic research, preclinical research, clinical trials, and regulatory approval are only a few of the phases that go into clinical translation.
A QUICK GUIDE TO CLINICAL TRANSLATION?
Clinical translation begins with basic research, which entails finding potential novel cures and treatments. The second step is preclinical research, which entails testing potential novel cures and treatments on animals and in the laboratory. The final step involves putting prospective novel cures and treatments to the test on humans. The fourth and final phase entails obtaining regulatory approval.
DOES CLINICAL TRANSLATION POSE A CHALLENGE?
Clinical translation is the process of converting discoveries made in fundamental research into fresh forms of medication and diagnostic equipment. It is crucial for the creation of novel therapeutics and for providing patients with cutting-edge medical treatments. Clinical translation can be a challenging process that involves a wide range of stakeholders, including fundamental researchers, doctors, pharmaceutical corporations, and regulatory organizations. For the purpose of discovering new disease mechanisms and potential therapeutic targets, basic research is crucial. However, in order for physicians to provide patients with better care, these discoveries must be turned into fresh therapies and diagnostic equipment. Since they are in charge of creating new medicines, pharmaceutical corporations play a crucial part in this process.